April Zhu |The New Humanitarian
Freelance journalist based in Nairobi, Kenya
‘Even where there is enough money, many African health authorities are unable to obtain the supplies needed.’
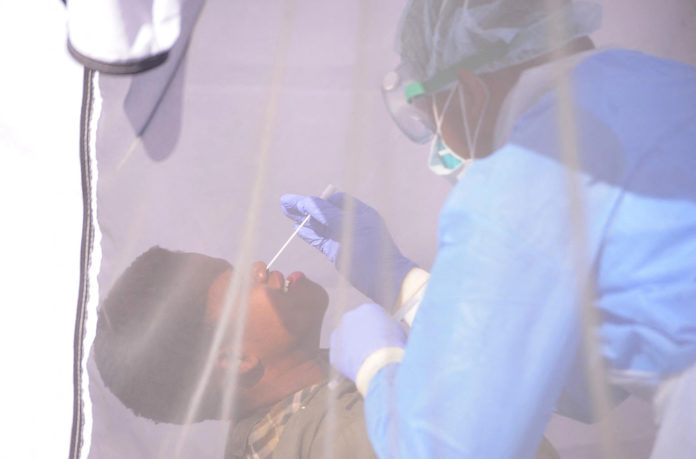
“Test, test, test” has been the mantra for defeating the novel coronavirus, but African countries are finding themselves at the end of a long global queue for the chemical reagents and other commodities necessary for administering diagnostic tests, according to public health experts.
What testing is being done shows a steep rise in COVID-19 cases. In the past three weeks alone, the numbers have nearly trebled – from 33,273 on 28 April to more than 85,000 on 18 May – according to figures from the Africa Centres for Disease Control and Prevention.
The latest modelling by the World Health Organisation predicts 29 million to 44 million Africans could be infected in the first year of the pandemic if containment measures fail – with 83,000 to 190,000 deaths.
Although mass testing is seen as a key component to slowing transmission, African health authorities are struggling to compete with richer, more powerful countries when it comes to procuring the scarce testing material on the global market, notes a new commentary published in The Lancet scientific journal.
“Even where there is enough money, many African health authorities are unable to obtain the supplies needed,” the commentary points out.
Testing dilemma
There is a significant divergence in testing performance among Africa’s 54 countries, as of 18 May.
In East Africa, while Uganda has conducted over 71,000 tests and Rwanda 48,200, neighbouring Tanzania has conducted only 652 (509 of which have come back positive) – which President John Magufuli has, controversially, attributed to faulty tests.
Read more → Tanzanian doctors sound alarm over hidden coronavirus cases
So far, South Africa and Ghana have accounted for nearly half of all tests carried out on the continent. Nigeria, Africa’s most populous nation of 200 million citizens, has conducted just 23,835 tests.
South Africa, by far the most aggressive tester, has administered over 439,500. The country has capitalised on existing networks of community and primary healthcare workers, relying on systems built to combat HIV and other infectious diseases.
“This infrastructure and experience with testing and tracing is an amazing experience to apply to COVID-19,” Ngozi Erondu, a senior research fellow at the Chatham House Centre for Global Health Security, and one of the authors of the Lancet commentary, told The New Humanitarian.
But even with its relatively advanced infrastructure and laboratory capacity – South Africa has leveraged a network of over 200 laboratories – testing centres are struggling with major backlogs. For South Africa to test one percent of its population – a feat only achieved by a few countries, like South Korea and Germany – it would need to double the number of tests so far undertaken.
Yet that is not beyond the human resource capacity of African countries, according to the Lancet paper. It says that for Tanzania to reach one percent of its population, it would need to test 597,342 people. But, in a three-month period in 2019, Tanzania performed well over 1.4 million HIV tests. Several African countries HIV tested more than four percent of their citizens over the same period.
The bottleneck is beyond political will; it is a practical problem – the difficulty African health authorities face in competing for supplies. The Lancet authors recognise tough decisions on resource allocation are inevitable, but, they note, “it is unethical for African countries to have considerably less access and harder choices than others.”
“This is not a question of demanding charity,” Africa CDC head John Nkengasong wrote in a sharply worded commentary for the scientific journal Nature. “African countries have funds to pay for reagents but cannot buy them.”
Even low-margin, low-tech items like swabs to take samples are in short supply on the international market.
“The collapse of global cooperation and a failure of international solidarity have shoved Africa out of the diagnostics market,” said Nkengasong, pointing to the fact that over 70 countries have restricted exports of medical goods.
“The collapse of global cooperation and a failure of international solidarity have shoved Africa out of the diagnostics market.”
The Lancet commentary warns against a repeat of the AIDS pandemic, when diagnostics and life-saving affordable drugs were available to African countries far later than those in the West. “Political leadership is urgently required to secure ethical global allocation of scarce resources,” it noted. “It is entirely foreseeable that many countries will be locked out of the market.”
Though Africa CDC has announced a plan to distribute one million test kits this month, it would only be a temporary solution for the sort of widespread testing and tracing necessary.
The WHO’s regional director for Africa, Dr. Matshidiso Moeti, has warned that there is a risk coronavirus could “smoulder in transmission hotspots” for years.
“COVID-19 could become a fixture in our lives for the next several years unless a proactive approach is taken by many governments in the region,” she said. “We need to test, trace, isolate, and treat.”
Building self-sufficiency
Many African researchers foresaw this scarcity and the consequent need for African countries to be as self-sufficient as possible when it comes to diagnostic tests.
Given the global shortages – particularly the reagents necessary to run polymerase chain reaction (PCR) tests, which remain almost exclusively the basis for diagnosis – African countries need to take two paths forward, suggests Dr. Misaki Wayengera of Uganda’s Makerere University.
The first strategy is to validate existing rapid diagnostic tests, or RDTs.
With the exception of some highly sensitive RDTs used in high-income countries, RDTs are largely not recommended by the WHO for use as the sole basis for COVID-19 diagnosis. That’s because instead of testing directly for the genetic material of the coronavirus, as PCRs do, they test for the body’s immunological response, and can give false-positives.
But Wayengera, who won international acclaim for his Ebola diagnostic test, said a rethink is needed. Uganda now has a protocol for validating RDTs, meaning demonstrating RDTs’ accuracy, reliability, and replicability using samples acquired from individuals in Uganda who tested positive via PCR.
“I think it is worthwhile trying to [validate existing RDTs], in our setting,” said Wayengera. “Because what is the other option in case we don’t have enough tests premised on the current WHO-accredited platforms?”
The second strategy is to develop successful RDTs on the African continent – to ensure local availability. Wayengera and his team are working on several diagnostic tests, one of which is antigen-based that depends on a more basic immunodiagnostic technology and reagents readily available in labs throughout Uganda.
Meanwhile, UK-based diagnostic manufacturer Mologic is partnering with the Institut-Pasteur Dakar in Senegal to develop an RDT. The test is currently undergoing validation and, if approved, would cost less than $1 and yield results in minutes.
But there are only a few diagnostics manufacturing facilities across Africa that have developed RDTs and could work on COVID-19 solutions, acknowledged Dr. Moses Alobo of the African Academy of Sciences, who is leading the effort to coordinate elite researchers across the continent.
“We would need RDT kits in the millions, maybe billions,” he said. “I don’t think they’re there yet.”